LIFE SCIENCES
Lattice Boltzmann Simulation of Hemodynamics in Cerebral Aneurysms
Principal Investigator:
Prof. Dr. Dominique Thevenin
Affiliation:
Otto von Guericke Universität, Magdeburg, Germany
Local Project ID:
pn73ta
HPC Platform used:
SuperMUC-NG PH1-CPU at LRZ
Date published:
Introduction
Intracranial aneurysms, affecting approximately 3% of the western population, pose a serious threat due to the risk of rupture, leading to irreversible disabilities or death. Leveraging the computational power of modern supercomputers, our project employs the lattice Boltzmann method (LBM) to delve into the complexities of hemodynamics within intracranial aneurysms, utilizing our in-house numerical solver, ALBORZ.
Our research addresses the challenge of complex geometry in patient-specific aneurysms by introducing a curved boundary condition, enhancing the accuracy of LBM simulations. Additionally, we propose a robust modified central Hermite polynomial-based multiple relaxation time lattice Boltzmann model for modeling blood flow, challenging assumptions about high shear rates within aneurysm sacs.
A crucial focus of this project lies in the exploration of unsteady flow and flow fluctuations within patient-specific aneurysms. Flow instability emerges as a promising hemodynamic metric for assessing rupture risk, and our study investigates these fluctuations using both Newtonian and non-Newtonian fluid models.
Our findings reveal intriguing insights, particularly in ruptured aneurysms where hydrodynamic instability during pulsatile inflow contributes to high-frequency fluctuations around the rupture location. Importantly, we observe minimal differences between Newtonian and non-Newtonian outcomes in unruptured cases but significant variations in ruptured cases.
This work significantly contributes to understanding hemodynamics in intracranial aneurysms, shedding light on the role of non-Newtonian behavior and flow fluctuations in assessing the rupture risk of these critical medical conditions.
Results and Methods
To validate the accuracy and reliability of our numerical solver (ALBORZ), we initially selected a patient specific aneurysm [2]. Figure 1 presents a qualitative comparison between the hemodynamic simulations based on the lattice Boltzmann (LB) approach and in-vitro phantom measurements. The agreement with particle image velocimetry (PIV) data indicates the solver's capability to accurately capture the flow structure, even in relatively under-resolved simulations.
Figure 1: From left to right: Qualitative comparison of the velocity fields acquired using PC-MRI (1st column), stereoscopic PIV (2nd column), instantaneous (3rd column) and time-averaged LBM (last column) simulation in the aneurysm sac on three different planes, i.e. from top to bottom: planes I, II and III.
For benchmarking, we employed the US Food and Drug Administration (FDA) suggested idealized medical nozzle device. The simulation, conducted on 1,500 cores in parallel, utilized a discretized domain of 155×155×3,093 equidistant grid points (74.3 million points). Figure 2 displays the results from the FDA nozzle simulation at Re = 6,500, revealing local turbulence downstream of the sudden expansion region, consistent with both PIV and numerical observations [3].
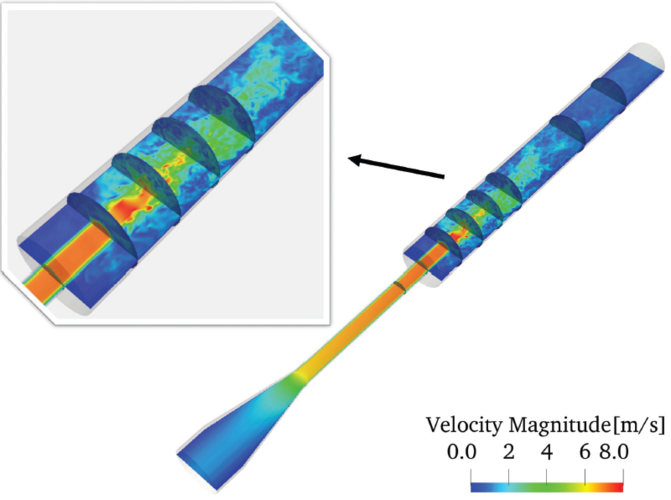
Figure 2: Re = 6,500. Instantaneous results obtained by LBM for the axial velocity along the centerline of the nozzle.
In addressing the challenging task of assessing the risk of rupture in intracranial aneurysms, we conducted direct numerical simulations on patient-specific cases. Both Newtonian and non-Newtonian fluid models were considered. The domains for the aneurysms were specified as 21.2 × 37.6 × 19.7 mm and 32.5 × 18.4 × 11.1 mm, with corresponding core hours of 8,760 and 3,810. Figure 3 provides a qualitative comparison of flow streamlines within the aneurysm sac, offering insights into dynamic behavior during different phases of the cardiac cycle. This initial qualitative analysis revealed a stronger flow with larger variations in time between ruptured and unruptured cases.
Figure 3: Flow streamlines colored by flow velocity inside the aneurysm sac.
In summary, our validation process and simulation results contribute to the robustness of ALBORZ and pave the way for a detailed investigation into the elusive criterion for assessing rupture risk in intracranial aneurysms.
Ongoing Research / Outlook
In the coming phases of our research, we aspire to elevate our numerical procedure to simulate an even broader spectrum of patient-specific cases with heightened accuracy. This evolution entails advancements in resolution, incorporation of realistic geometries, and a nuanced consideration of additional physiological factors. By doing so, we aim to extend our analyses to a more extensive dataset, cultivating a comprehensive understanding of aneurysm behavior that transcends the limitations of smaller datasets.
Furthermore, our future study will extend to encompass numerical simulations involving stents placed within aneurysms. This expansion is motivated by the need to evaluate the effectiveness of existing and emerging medical devices for aneurysm treatment. By simulating the interaction between stents and blood flow, we aspire to assess device stability, discern hemodynamic changes, and explore opportunities for optimization.
In summary, our future research directions aim to not only enhance then accuracy and scope of our simulations but also to explore new frontiers in medical device evaluation within the context of intracranial aneurysms.
References and Links
[2] S.A. Hosseini et al., Computers in Biology and Medicine 131(7):104251 (2021).
[3] F. Huang et al., Computer Methods and Programs in Biomedicine 221(1-3):106863 (2022).