LIFE SCIENCES
Molecular Mechanisms leading to the Desensitization of Nicotinic Acetylcholine Receptors
Principal Investigator:
Prof. Dr. Holger Gohlke
Affiliation:
Institute for Pharmaceutical and Medicinal Chemistry, Heinrich Heine University Düsseldorf, 40225 Düsseldorf, Germany
Local Project ID:
nAChR
HPC Platform used:
JUWELS Booster module of JSC, NIC
Date published:
Teaser
Prof. Dr. Holger Gohlke and Jesko Kaiser investigated the binding of resensitizers in the nicotinic acetylcholine receptor as a potential treatment option for nerve agent poisoning. They identified a potential allosteric binding site, explaining the experimentally observed effect on the receptor. Based on these results, the researchers identified novel analogs with improved properties and new lead structures with improved affinity compared to MB327, potentially acting as new starting points to ultimately close the gap in nerve agent poisoning treatment.
Project
Despite international efforts to ban organoposphorous compounds (OPCs) as chemical warfare agents, the compounds are still being used - for example, for assassination attempts of opposition politicians and during the Syrian civil war. Due to covalent inhibition of the acetylcholine esterase they lead to an overstimulation of nicotinic acetylcholine receptors (nAChRs), which leads to desensitization of those receptors, associated with severe symptoms, such as respiratory paralysis, that can lead to death. The current treatment of OPC poisoning is insufficient with no drug targeting the nAChR directly. Recent research led to the development of two novel allosteric modulators of the nAChR, MB327 and UNC0646, showing resensitizing effects on the receptor. However, due to the low activity and tight therapeutic index, those compounds cannot be used in the treatment of OPC poisoning yet. Before this funding period started, it was known that those compounds bind to an allosteric binding site, but the exact location remained elusive, making structure-based ligand optimization approaches demanding.
In this project, Prof. Dr. Holger Gohlke and Jesko Kaiser present a potential allosteric binding site, MB327-PAM-1, of MB327 and UNC0646 within the nAChR that can transmit the experimentally observed allosteric effects. Furthermore, based on these results, they identified several analogs of MB327 and UNC0646, displaying increased affinity towards MB327-PAM-1, and identified four new lead structures with improved affinity compared to MB327, including cycloguanil, the active metabolite of an approved antimalaria drug.
The team generated comparative models of the investigated receptor using Modeller. Using these models, the researchers performed blind docking experiments to identify a potential allosteric binding site of MB327. To consider receptor plasticity and assess the stability of the proposed binding mode, MD simulations were performed based on the blind docking results. During these simulations, important interaction partners were identified, and the allosteric impact of MB327 on nAChR was investigated. The ligand displays an allosteric effect on the orthosteric binding site and the desensitization gate. This is in line with experimental results that the ligand leads to an enhanced cholinergic effect and acts as a resensitizer of the receptor. To investigate whether UNC0646 may also bind to MB327-PAM-1, the team performed flexible docking experiments, combined with MD simulations to highlight that amino acids important for the stabilization of MB327 also act as interaction partners of UNC0646.
To identify new analogs of the known compounds, the team performed a variety of experiments. First, novel analogs were suggested based on the visual inspection of the binding site and investigation of potential new interactions of the nAChR with those analogs. Thereby, two analogs of MB327 were identified, displaying increased activity compared to MB327. Next, the team analyzed entropically unfavorable water molecules within the binding site to suggest variations that would result in replacing those water molecules, leading to an analog with increased affinity compared to MB327. In another approach, the researchers performed ligand-based drug design experiments to identify analogs of UNC0646 with increased affinity.
Furthermore, the team identified a total of four novel analogs (Figure 1), all featuring increased affinity towards MB327-PAM-1 compared to MB327. Notably, one of those compounds, cycloguanil, is the active metabolite of an already approved and well-tolerated antimalaria drug. This compound was also tested for its resensitizing properties after soman poisoning of rat muscles. Compared to MB327, the compound shows the same maximum reestablishment of the muscle force but generates the maximum at lower concentrations, in line with its increased affinity. However, because of the tight therapeutic index of cycloguanil, this compound cannot be considered a treatment option yet but can be used as a new lead structure for further drug optimization.
In summary, the team’s effort resulted in the identification of the potential allosteric binding site of MB327 and UNC0646 in nAChRs. Based on these results, rational drug design became feasible, resulting in a variety of improved analogs of known ligands. Furthermore, the researchers were able to identify four novel lead structures with improved properties compared to MB327.
Publications
In the granting period, the following articles were published:
J. Kaiser, C.G.W. Gertzen, T: Bernauer, G: Höfner, K.V. Niessen, T. Seeger, F.F. Paintner, K.T. Wanner, F. Worek, H. Thiermann, H. Gohlke, A novel binding site in the nicotinic acetylcholine receptor for MB327 can explain its allosteric modulation relevant for organophosphorus‐poisoning treatment, Toxicol Lett, 2023. 373: p. 160-171.
V. Nitsche, G. Höfner, J. Kaiser, C.G.W. Gertzen, T. Seeger, K. V. Niessen,
D. Steinritz, H. Thiermann, F. Worek, H. Gohlke, F.F. Paintner, K.T. Wanner, MS Binding Assays
with UNC0642 as reporter ligand for the MB327 binding site of the nicotinic acetylcholine
receptor, Toxicol Lett, 2024. 392: p. 94-106.
J. Kaiser, C.G.W. Gertzen, T. Bernauer, V. Nitsche, G. Höfner, K.V.
Niessen, T. Seeger, F.F. Paintner, K.T. Wanner, D. Steinritz, F. Worek, H. Gohlke,
Identification of ligands binding to MB327-PAM-1, a binding pocket relevant for
resensitization of nAChRs; Toxicol Lett, 2024. in press.
T. Bernauer, V. Nitsche, J. Kaiser, C.G.W. Gertzen, G. Höfner, K.V.
Niessen, T. Seeger, D. Steinritz, F. Worek, H. Gohlke, K.T. Wanner, F.F. Paintner,
Synthesis and Biological Evaluation of Novel MB327 Analogs as Resensitizers for
Desensitized Nicotinic Acetylcholine Receptors after Intoxication with Nerve Agents,
Toxicol Lett, 2024. 397: p. 151-162.
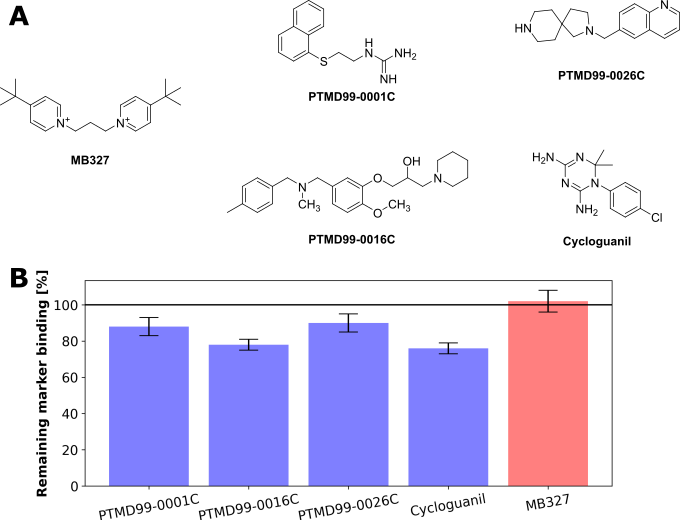
Figure 1: The identified novel lead structures that can act as starting points for drug optimization. A) Structure of MB327 and the newly identified lead structures within this funding period, PTMD99-0001C, PTMD99-0016C, PTMD99-0026C, and cycloguanil. B) Comparison of the remaining marker binding of the reporter ligand UNC0642 (an analog of UNC0646, concentration during experiments 1 μM) during MS Binding Assays after adding the respective ligand (concentration during experiments 10 μM). Because substitution of the reporter ligand correlates with the affinity of each compound, lower values indicate an improved affinity.