MATERIALS SCIENCE AND CHEMISTRY
Let There be Light: The Case of the Major Light Harvesting Complex II
Principal Investigator:
Vangelis Daskalakis
Affiliation:
Cyprus University of Technology
Local Project ID:
pn34we
HPC Platform used:
SuperMUC-NG of LRZ
Date published:
Introduction
In our lab, we probe the biophysics of Photosynthesis [1]. Photosynthesis fuels the metabolic pathways of numerous organisms in our biosphere. This is achieved by storing the solar energy into chemical bonds and by contributing to the atmospheric oxygen cycle. However, the diurnal cycle, or the environmental conditions induce fluctuations in the light intensity or quality, and thus they render it unreliable as a source of energy [2]. Higher plants have to cope with such fluctuations to sustain their homeostasis; too little light and photosynthesis cannot occur, too much and the photosynthetic apparatus is at risk of damage, due to oxidative stress [2]. Absorption of light and tunnelling of the associated energy towards the reaction centres of the photosynthetic apparatus are finely-tuned in this context.
The photosynthetic apparatus has a central component: the major Light Harvesting Complex (LHCII) of Photosystem II (PSII). In green plants, LHCII is found as a trimer of polypeptide scaffolds with densely packed networks of pigments within (chlorophylls and catorenoids). LHCII is able (a) to capture the solar energy under normal light conditions, and (b) to quench the excess energy when the light absorption exceeds the capacity of the reaction centres (Fig. 1).
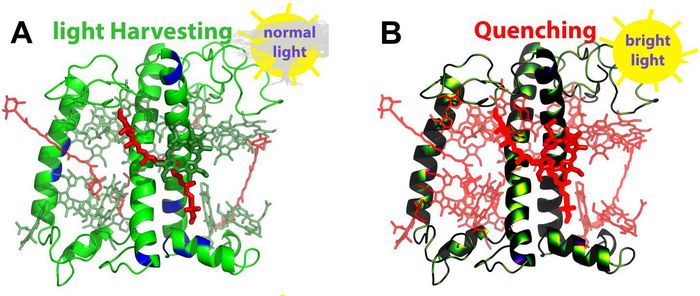
Figure 1: The chain C monomer of the major Light Harvesting Complex II (LHCII) is shown in green-blue cartoons (A). Chlorophylls are shown in green and carotenoids in red licorice. Under normal light, pigments absorb the solar energy and direct it to the reaction center of Photosystem II. Under excess light, LHCII switches to a quenched state where the absorbed solar energy in excess is dissipated as heat (B). Blue regions on the cartoons of A indicate important residues for the switch [3]. © Cyprus University of Technology
LHCII within the thylakoid membrane has the potential to rapidly and reversibly switch between these roles via elusive conformations. At the quenched state, the absorbed energy in excess can be thermally dissipated under mechanisms that are cumulatively referred to as nonphotochemical quenching (NPQ) of Chl fluorescence [2].
Several factors have been associated with NPQ: the increase of the transthylakoid membrane proton gradient (ΔpH) by lumen acidification, ion flows in the lumen-stromal areas, changes in the carotenoid content of LHCII from Violaxanthin to Zeaxanthin by the xanthophyll cycle, and finally the photoprotective protein PsbS that binds to LHCII, and can induce LHCII aggregation [4].
Research into these factors has led to the development of more efficient systems in the field of artificial photosynthesis and the increase of crop yields. Our project has focused on the LHCII-PsbS interaction that affects (i) the LHCII aggregation within the thylakoid membranes of higher plants and (ii) the interpigment interactions within LHCII induced by LHCII conformational transitions [3,5].
Results and Methods
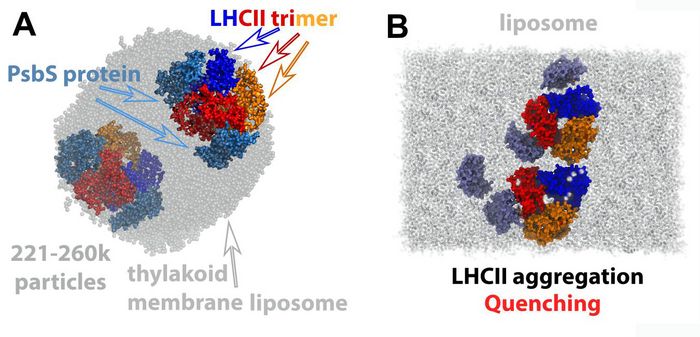
Several LHCII-PsbS models were built at all-atom (AA) resolution [3], and at the coarse-grain (CG) level [5]. These were embedded within a thylakoid membrane patch, or a thylakoid liposome (Fig. 2). We have proposed that in the transition of the LHCII trimer from the light harvesting to the quenched state, a change in the shape of the protein is induced, that is associated with a thinning of the thylakoid membrane, also observed experimentally [5]. The change in the LHCII shape induces an accumulation of galactolipids around LHCII to lower the cost of hydrophobic mismatch. When PsbS is activated, it binds to LHCII proteins, the galactolipids around LHCII are expelled and thus LHCII is aggregating due to solvent (lipid) depletion. The proposed mechanism clearly explains why an enhanced pH gradient (ΔpH) is regulating the photosynthetic process. PsbS is able to control the LHCII trimer diffusion and aggregation within the thylakoid membranes through the manipulation of the induced hydrophobic mismatch at enhanced ΔpH [5]. The LHCII-PsbS models at AA resolution, along with numerous others, have also provided the complete computational framework to drive the LHCII conformation between, previously elusive, different states, associated with light harvesting and quenched modes [3].
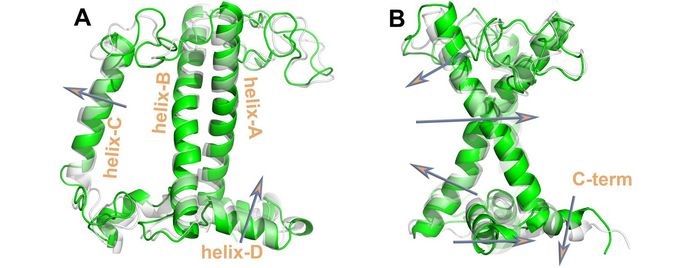
Thus, we have probed for the first and at the same time the effects of all the key NPQ factors, on the LHCII trimer conformation. The NPQ related LHCII main conformational transitions reported (Fig. 3) are in line with the NPQ literature. However, even with the extensive sampling reported herein for the protein scaffold transitions, a hard switch between hight harvesting and quenching is still absent in the induced interpigment interactions probed by a common approach in computational chemistry [3]. Thus, this study has shifted the attention from the extensive conformational sampling, to the problem of accurately describing interpigment
interactions within LHCII (i.e. by accurate ab initio methods) [3].
For this project, elaborate computational methods were employed to achieve our goals described in detail elsewhere [3,5]. In brief, PTmetaDWTE, or Parallel Tempering Metadynamics Simulations were employed at the Well-tempered Ensemble (a variant of Replica Exchange) to probe how LHCII trimers (CG) aggregate within the thylakoid membrane of higher plants, or how LHCII conformation is altered upon LHCII-PsbS interaction at AA resolution (Fig. 3). A cumulative sampling of more than 150μs at AA/CG levels of LHCII models was made possible by the SuperMUC-NG allocated resources (17 million core hours). This has yielded a total of around 210 GB production data in around five hundred files for analysis. A typical job was run on 3,072 cores.
Ongoing Research / Outlook
The gained understanding of LHCII-PsbS interaction and LHCII aggregation can further be exploited to guide future experimental or computational studies on plant photoprotection. We thank PRACE for awarding us access to the petascale HPC system SuperMUC-NG.
The work was cofunded by the European Regional Development Fund and the Republic of Cyprus through the Research and Innovation Foundation (Project: POSTDOC/0916/0049). Without the SuperMUC-NG resources, the extensive sampling by the PTmetaDWTE method would not have been possible. The main limitations/obstacles for this study were the convergence of the results, which we overcame by the most extensive sampling of such systems up to date (150μs). In addition, the important shift in focus for the research in NPQ, that we proposed (from conformational sampling to accurately describing interpigment
interactions), would be impossible to predict without the allocated amount of resources.
SuperMUC-NG could be employed for future studies to describe such interpigment interactions by ab initio methods. The setup of such simulations is under way, along with proposals for submission to future PRACE/DECI calls. Unpublished results from this project (mainly related to the LHCII-lipid interactions in liposomes) are still under preparation for future publications.
Research Team
Vangelis Daskalakis1, Eleni Navakoudis1,2, Sotiris Papadatos1, Taxiarchis Stergiannakos1
1Cyprus University of Technology
2University of Crete
References and links
[1] CEM lab. https://cemlab.wordpress.com/.
[2] Ruban, A. V, Johnson, M. P. & Duffy, C. D. P., Biophys. Acta (BBA)Bioenergetics 1817, 167–181 (2012).
[3] Daskalakis, V., Papadatos, S. & Stergiannakos, T., Chem. Commun. (2020) doi:10.1039/D0CC04486E.
[4] Ruban, A. V. Light harvesting control in plants. FEBS Lett. 592, 3030–3039 (2018).
[5] Daskalakis, V., Papadatos, S. & Kleinekathöfer, U., Biochim. Biophys. Acta Biomembr. 1861, 183059 (2019).
Scientific Contact
Prof. Dr. Vangelis Daskalakis
Cyprus University of Technology
Department of Chemical Engineering
30 Arch. Kyprianos Str.
3036 Limassol (Cyprus)
e-mail: evangelos.daskalakis [@] cut.ac.cy
https://www.researchgate.net/profile/Vangelis-Daskalakis
NOTE: This simulation project was made possible by PRACE (Partnership for Advanced Computing in Europe) allocating a computing time grant on GCS HPC system SuperMUC-NG of the Leibniz Supercomputing Centre (LRZ) at Garching near Munich, Germany. GCS is a hosting member of PRACE.
Local project ID: pn34we
February 2021