MATERIALS SCIENCE AND CHEMISTRY
Composition and Structure of β-Ga2O3(001) under Realistic (T, p) Conditions
Principal Investigator:
Prof. Claudia Draxl
Affiliation:
Institut für Physik and IRIS Adlershof, Humboldt-Universität zu Berlin, Germany
Local Project ID:
pn29ji
HPC Platform used:
SuperMUC-NG at LRZ
Date published:
Introduction
This work was performed in the framework of GraFOx, a Leibniz-ScienceCampus partially funded by the Leibniz association [1].
Gallium oxide (Ga2O3), a transparent semiconducting oxide with a wide bandgap of around 4.9 eV, has emerged as a promising candidate for future applications in electronics (Schottky barrier diodes, field-effect transistors), optoelectronics (solar- and visible-blind photodetectors, flame detectors, light emitting diodes, touch screens), and sensing systems (gas sensors, nuclear radiation detectors) [2]. The monoclinic β phase is its most stable and studied polymorph. Compared to the bulk properties, research on its surface properties is still sparse. However, these play a crucial role in many processes and applications, such as epitaxial growth and electrical contacts. We have previously shown that surface stability is critical to understanding faceting on β-Ga2O3 substrates [3].
Of particular importance is the occurrence of surface reconstructions in reactive environments and under realistic temperature and pressure conditions, which may affect electrical properties and surface stability. To this end, we have applied the recently developed Replica-Exchange (RE) Grand-Canonical (GC) method [4] in conjunction with ab initio molecular dynamics (MD) simulations to obtain an accurate temperature-pressure phase diagram of the β- Ga2O3(001) surface in a reactive O2 gas phase. Compared to the conventionally used ab initio atomistic thermodynamics (aiAT) method, this approach is not only less biased but also includes vibrational contributions with full anharmonicity.
Results and Methods
We consider several replicas of the surface system, each evolving in a different thermodynamical state. During the simulation, each replica has a certain probability to either exchange particles with the oxygen gas phase or perform a RE step. After the particle/RE step, the surface system is diffused in the canonical ensemble via MD simulations. REGC-MD is massively parallel by design, as the replicas communicate only during the RE steps, allowing full use of the SuperMUC-NG architecture. The main cost of the REGC-MD method is associated with the ab initio MD simulations. The MD simulations to properly diffuse all replicas were performed at the density-functional theory level with the state-of-the-art all-electron code FHI-aims [5]. It employs a numeric atom-centered orbital basis set that allowed us to add large amounts of vacuum at minimal computational cost. We simulated the surfaces in slab supercells of up to 360 atoms. We considered 60 replicas, with each replica using 10 full nodes (480 cores). Such large and involving calculations were only possible on a Tier 0 computing center. In total, we produced more than 300 GB of data throughout the project.
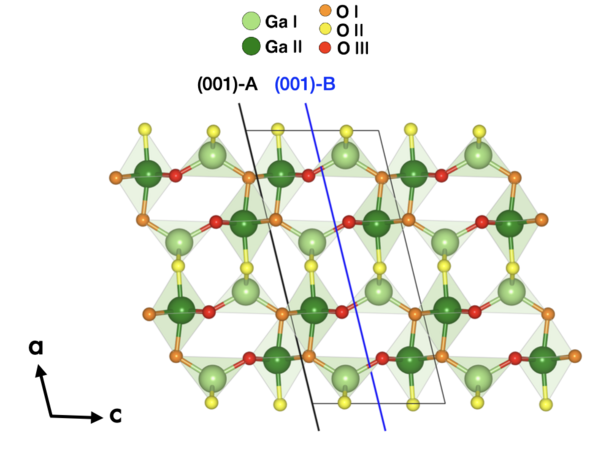
Figure 1: The conventional bulk cell of β-Ga2O3. The inequivalent gallium atoms are in light and dark green, while the inequivalent oxygen atoms are orange, yellow, and red. The two stoichiometric (001) terminations, (001)-A and (001)-B, are indicated by the black and blue lines.
There are two stoichiometric terminations for the (001) surface, (001)-A and (001)-B, as shown in Fig. 1. The coordination environment at the surface is significantly different for the two terminations. The B termination is much more stable, as only the octahedrally coordinated Ga(II) atoms are under-coordinated, while the tetrahedrally coordinated Ga(I) atoms are under-coordinated in (001)-A. Both terminations occur naturally during growth.
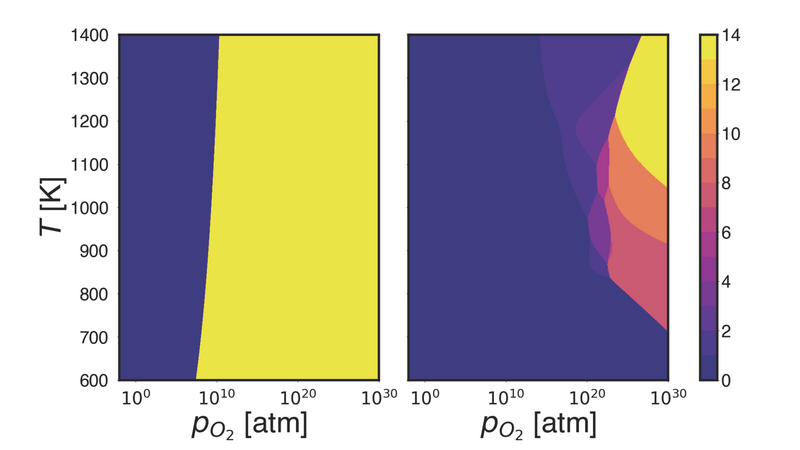
Figure 2: Shown are the phase diagrams of β-Ga2O3 (001)-B in contact with a reactive oxygen atmosphere obtained with ab initio atomistic thermodynamics (left) and REGC-MD after 1000 steps (right). The colors correspond to the number of adsorbed oxygen atoms in the slab.
We obtained a converged phase diagram for the (001)-B surface, which is given in Fig. 2, where we also compare our results with the more commonly used aiAT approach. The differences are evident. The bare surface and the phase with 14 adsorbates show a region of stability in the aiAT phase diagram. Only by including the full vibrational contributions to the free energy with REGC-MD can we find additional stability windows for several phases. The vibrational contributions also shift the stability window of the bare surface to much higher partial pressures at low temperatures (<1000 K) than given by aiAT. The bare (001)-B surface was remarkably stable throughout the simulation. Oxygen atoms adsorbed preferably on the Ga (II) site, where they then typically formed a bridge-like bond to the O (I) site. However, no significant reconstruction of the surface occurred during the simulation. This suggests that other surfaces with only under-coordinated Ga (II) sites, such as β-Ga2O3(100)-B, will also not reconstruct.
We also obtained a preliminary phase diagram for the (001)-A surface. The results are shown in Fig. 3. When including all vibrational effects with REGC-MD, the bare surface is still stable for much higher partial pressures p < 1011 atm. In addition, we can see the emergence of several metastable structures. Interestingly, we find that the relaxed bare (001)-A surface is actually not stable at the temperatures considered here. It reconstructs by letting under-coordinated Ga (I) regain their tetrahedral coordination environment. As a result, we find an alternating sequence of tetrahedra-octahedra-tetrahedra at the surface that differs significantly from the bulk environment. When the environment is oxygen-rich, oxygen atoms typically adsorb on the Ga (I) site and prevent the formation of the reconstruction. The structure (001)-A+O2 with two additional adsorbed oxygen atoms exhibits a large stability range in the phase diagram up to a partial pressure of p < 1016 atm. It represents a previously unknown 1/2x1 reconstruction of the (001)-A surface. Here both the dangling bonds of Ga (I) and O (III) are partially satisfied following the adsorption.
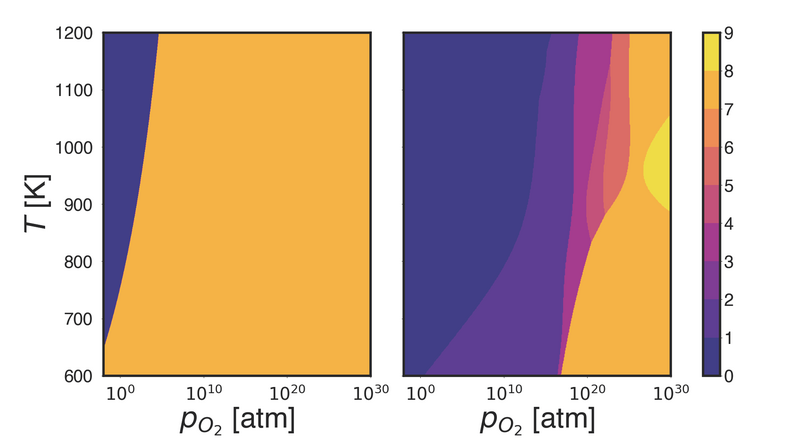
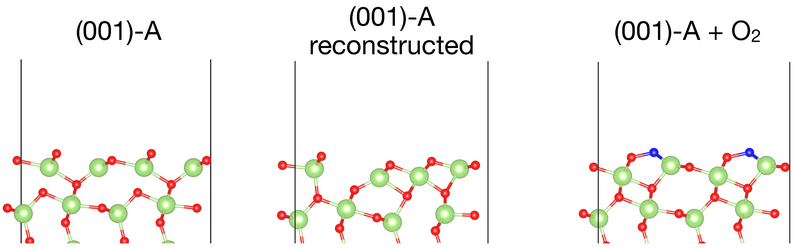
Figure 3: Top: Shown are the phase diagrams of β-Ga2O3 (001)-A obtained with ab initio atomistic thermodynamics (left) and REGC-MD after 500 steps (right). The colors correspond to the number of adsorbed oxygen atoms in the slab. Bottom: The unreconstructed bare (001)-A surface (left), the reconstructed bare (001)-A surface (middle), the 1/2x1 reconstruction with two adatoms (right). Gallium atoms are green, oxygen atoms are red, and additional oxygen adatoms are blue.
We have shown that we can obtain an accurate phase diagram only if we take into account the full vibrational contributions to the free energy. Our results further indicate that the (001)-B termination is extremely stable even in very oxygen-rich conditions. However, we have found (at least) two possible reconstructions of the (001)-A surface. These results show that we can predict accurate phase diagrams and surface reconstructions of complex surfaces, without prior knowledge or bias.
Ongoing Research / Outlook
One limiting factor of our current approach is that we only simulate an environment with oxygen. During the growth of β-Ga2O3 both oxygen and gallium are available. We are currently trying to generalize the REGC-MD method to consider more than one reactive gas in the so-called constrained equilibrium, where different species do not react in the gas phase but only at the surface. In addition, all the data obtained can be reused in subsequent studies. The combination of the REGC sampling and a posteriori analysis allows for the determination of phase diagrams for any (atom position dependent) observables, which provide information on how to adjust the environmental conditions to obtain a material with the desired properties.
References and Links
[2] Z. Galazka, Semiconductor Science and Technology 33 (2018), 113001.
[3] R. Schewski et al., APL Materials 8 (2019), 022515
[4] Y. Zhou, M. Scheffler, L. M. Ghiringhelli, Phys. Rev. B 100 (2019), 174106.
[5] V. Blum et al., Comput. Phys. Commun. 180 (2009), 2175-2196