MATERIALS SCIENCE AND CHEMISTRY
Dynamics of Complex Fluids - Electrokinetics and Electrohydrodynamics
Principal Investigator:
Jens Harting
Affiliation:
HI-ERN, Forschungszentrum Jülich GmbH (Germany)
Local Project ID:
chfz05
HPC Platform used:
JUWELS of JSC
Date published:
Antagonistic salts have the special property that in a mixture of water and oil (or generally polar and unpolar fluids) one ion type will migrate into the water phase while the oppositely charged ion type will go to the oil phase. This unusual behaviour is triggered by a substantial size difference between the anion and the cation in an antagonistic salt. Smaller ions are easily solvated by water while larger ions tend to be squeezed out of the water phase due to the strong hydrogen bonds between water molecules.
Recent experimental studies of the antagonistic salt sodium tetraphenylborate (NaBPh4), in a mixture of water and 3-methylpyridine (3MP), show that this ion separation can trigger the formation of highly regular, nanometer scale structures consisting of alternating regions of high water+Na+ concentration and high 3MP+BPh- concentration. Such mixtures even change colour with a strong temperature sensitivity when the size of these nanostructures is of the order of the wavelength of visible light.
In the coarse-grained model of the system two fluids are initialized in a mixed state and proceed to demix, like water and oil might, due to a simple repulsive force. On the same lattice as the fluid positive and negative ion concentrations evolve due to diffusion, electrostatics and an additional solvation potential, which drives the positive ions into the water phase and the negative ions into the oil phase. The resulting opposite charges formed in the two fluids cause an attractive electrostatic force between the water and oil phases that counteracts the demixing force.
Depending on a number of system parameters such as the strength of the solvation potential, the total ion concentration, and the mixing ratio of water to oil, demixing is arrested with the minority phase forming either droplets, tubes or lamellae, as shown in Figure 1. The group found that the structure and its characteristic size obtained for a given set of parameters can be predicted using the same theory originally developed by T. Ohta and K. Kawasaki in 1986[1] to model mixtures of diblock copolymers.
Furthermore, when the water/oil ratio is 50/50 and lamellae are formed, the electrostatic interaction was found to drive a nematic, i.e. parallel ordering of the lamellae, which can be shown to minimize the free energy of the system and progress at a speed proportional to the initial concentration of ions.
Employing molecular dynamics simulations of the same atomistically resolved antagonistic salt NaBPh4 used in experiments, the group confirmed that the size difference of the ions triggers the same sort of separation into the water and oil (3MP) phases as enforced by the solvation potential in the coarse-grained model. Similar droplet and tubular structures were obtained, as shown in Figure 2, albeit at a smaller scale than in the coarse-grained model due to the high computational cost of atomistic simulations.
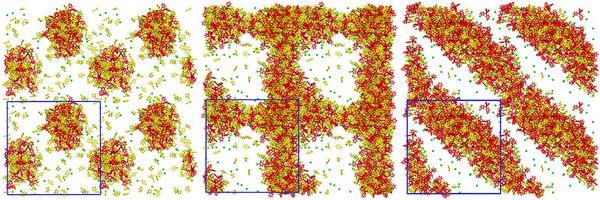
Figure 2: Shown are 3-methylpyridine (yellow), tetraphenylborate (red), and sodium (green) molecules/ions which formed similar structures to Figure 1 at water+Na+/3MP+BPh4- ratios of 85/15 (l) and 70/30 (m,r). The blue boxes delineate the boundaries of the simulated system surrounded by periodic copies. © HI-ERN
In the 50 000 atom simulations shown in Figure 2, the limited system size as compared to the size of the formed structures can be expected to strongly influence the result through the, in principle artificial, periodic boundary conditions. This is most likely the reason why different structures were obtained for similar concentrations in the two (roughly) 70/30 ratio pictures in Figure 2. Additional 1 million atom simulations for a select few points in parameter space, as shown in Figure 3, made it possible to verify, that similar structures result in the atomistic model as in the coarse-grained model when using similar mixing ratios.
The results on the coarse-grained model were published in 2019[2]. The presented results on the atomistic model, complemented by some additional results on how the lamellar phase can occur also for non-symmetric mixing ratios in the atomistic model but not in the coarse-grained model, will be submitted soon.
References and Publications
[1] T. Ohta, and K. Kawasaki, Equilibrium morphology of block copolymer melts, Macromolecules, 19, no. 10, 2621, Oct. 1986
[2] D. Jung, N. Rivas, and J. Harting, How antagonistic salts cause nematic ordering and behave like diblock copolymers, The Journal of Chemical Physics, 150, no. 6, 064912, Feb. 2019.
Scientific Contact
Prof. Dr. Jens Harting
Forschungszentrum Jülich GmbH
Helmholtz Institute Erlangen-Nürnberg (HI-ERN) for Renewable Energy
Fürther Straße 248, D-90429 Nürnberg (Germany)
e-mail: j.harting [@] fz-juelich.de
www.hi-ern.de/hi-ern/CompFlu/node.html
JSC project ID: hfz05
March 2020