MATERIALS SCIENCE AND CHEMISTRY
Embedding Approach to Hot Adatom Motion
Principal Investigator:
Karsten Reuter
Affiliation:
Lehrstuhl für Theoretische Chemie, Technische Universität München
Local Project ID:
pr85wa
HPC Platform used:
SuperMUC of LRZ
Date published:
Exothermic surface chemical reactions may easily release several electron volts of energy. Fundamental questions regarding the conversion and dissipation of this microscopically sizable amount of energy are critical in e.g. present day energy production and pollution mitigation, and yet in many cases remain unanswered. Scientists of the TU München promote microscopic understanding through a novel multi-scale approach which, for the first time, allows to model energy dissipation into substrate phonons from first-principles. The computationally demanding quantum-mechanical description of a “hot” reaction zone is only made possible through high-end HPC resources, provided by SuperMUC. It elucidates the complex interaction dynamics of adsorbates with phonons under non-equilibrium conditions and offers unprecedented insight towards accommodating such “hot chemistry”, for example, in our current understanding of heterogeneous catalysis.
Due to its central role in technologically relevant processes such as heterogeneous catalysis, sensing, corrosion or epitaxy, the adsorption and dissociation of molecules on solid surfaces has been a most active field of research for many decades. When breaking things down to elementary processes on the molecular scale, however, our understanding of such phenomena remains rather limited. At present this receives even further stimulus from the basic energy science perspective which adds fundamental questions like the conversion of energy forms at interfaces to the agenda.
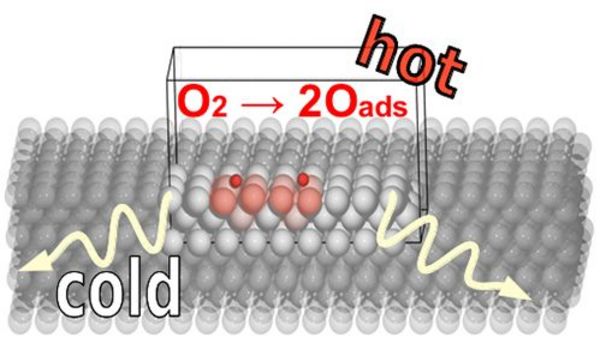
A particular example is the conversion of chemical energy to heat which arises as a consequence of a molecule's exothermic interaction with the surface and may easily amount to several electron volts of energy. For a prototypical model reaction like the dissociative adsorption of O2 molecules at metal surfaces, scanning-tunneling microscopy experiments have suggested the formation of so-called “hot” adatoms, i.e. dissociation fragments for which delayed heat dissipation results into a kinetic energy large enough to travel on the surface even at temperatures where thermal motion is prohibited.
As the experimental quest to generate molecular movies of such reactions is still ongoing, theory has been challenged to elucidate a full picture of the equilibration dynamics through computationally demanding quantum mechanical (QM) treatments [1,2]. Within the present Gauss project we focus on energy dissipation into phonons of the underlying metal (Me) substrate. We advance and apply a novel embedding scheme (QM/Me [3]) in which energy is dissipated out of a QM-described reaction zone and into a computationally undemanding, yet reliably described, extended bath. We thus scrutinize the complex adsorbate-phonon interaction dynamics beyond the harmonic approximation and under non-equilibrium conditions, while embracing the intriguing propositions brought forth by such “hot chemistry.”
In the application to O2 dissociation over Pd(100) QM/Me predicts “hot” dissociation fragments traveling ballistically over several lattice constants as a consequence of non-immediate energy transfer to the underlying surface. The interfacial conversion of energy and its dissipation to the bulk occurs within a few picoseconds after the initial O2 bond dissociation, thus indicating that the thermalization process is not instantaneous on the time scale of the elementary process itself and clearly influences the actual adsorbate dynamics. The ensuing transient mobility thus intricately couples the elementary reaction steps of dissociation and diffusion; a notion hitherto not considered in prevalent kinetic models in catalysis
High-end HPC resources provided by SuperMUC of the LRZ additionally allow for extending our investigation to several different systems, and further advancing our approach to encompass multiple reaction zones that can dynamically follow the “hot” adatom motion over an extended surface area [4]. This provides trend understanding for different substrates and surface symmetries, while also allowing the comparison to experimentally studied systems. A detailed atomistic understanding of the influence of surface temperature and underlying phonon dynamics is thus finally in reach, paving the way into unprecedented insight towards accommodating such “hot chemistry”, for example, in our current understanding of heterogeneous catalysis and energy conversion mechanisms at interfaces in general.
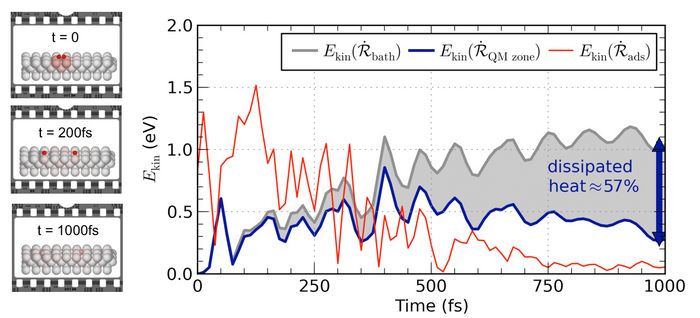
Fig. 1: Left: Snapshots along a dynamical trajectory depicting the motion of “hot” oxygen adatoms on a Pd(100) surface (color-coded to kinetic energy). Right: Profiles of kinetic energy as a function of time. Note that already within 1 picosecond after the initial O2 bond dissociation, phonons have propagated >50% of the initially released chemical energy outside the QM-described embedding cell and into the substrate bath. © Theoretische Chemie, TU München
References and links:
[1] V.J. Bukas, S. Mitra, J. Meyer and K. Reuter, J. Chem. Phys. 143, 034705 (2015)
[2] S. Rittmeyer, J. Meyer and K. Reuter, Phys. Rev. Lett. 115, 046102 (2015)
[3] J. Meyer and K. Reuter, Angew. Chem. Int. Ed., 53, 4721 (2014)
[4] V.J. Bukas and K. Reuter, in preparation.
Scientific contact:
Vanessa Jane Bukas
Theoretische Chemie, Technische Universität München
Lichtenbergstraße 4, D-85747 Garching/Munich
e-mail: vanessa.bukas (at) ch.tum.de
http://www.th4.ch.tum.de
LRZ Project ID: pr85wa
November 2015