MATERIALS SCIENCE AND CHEMISTRY
H2O@RUNG5 - Enabling the Next Level of Accuracy in First Principles Simulations at Finite Temperature: Double Hybrid DFT and RPA Simulation of Bulk Liquid Water
Principal Investigator:
Joost VandeVondele
Affiliation:
ETH Zürich
Local Project ID:
PP12071273
HPC Platform used:
Hermit of HLRS
Date published:
Water is called the substance of life: it covers most of the planet, it constitutes the largest fraction of our body, we drink it every day. Water actively participates in most chemical processes in nature, and will be the source of Hydrogen as we transition to clean and renewable energy. Surprisingly, and despite its abundance and importance, water is still poorly understood. In fact, more than 100 anomalous properties of water are known, water ice floating on liquid water being the most eye-catching one. The origin of the complexity is the subtle forces between water molecules, which derive from electrostatic, repulsive, hydrogen bonding, and van der Waals interactions. Getting the balance between these forces right is key for all of soft matter, and the sensitivity of water makes it the ideal testcase.
Computing the interactions between electrons and atoms requires solving the laws of quantum mechanics, the Schrödinger equation, accurately enough to capture the fine features. For almost 100 years this has been a very active field of research, in which supercomputing is essential. In fact, the equation is computationally so demanding to solve, that even on the largest supercomputers only approximate solutions can be obtained. However, the resources available at HLRS gave the team lead by Prof. VandeVondele (ETH Zürich) the opportunity to make a significant step forward, enabling the next level of accuracy in such simulations. In particular, computational approaches that treat van der Waals interactions at equal footing with the other forces have been applied to bulk liquid water for the first time. Compared to more standard approaches, these theories require roughly 1000 times more computational power, and have only become possible with significant progress in hard- and software development. [1,2] Interestingly, the weak interactions they describe make all the difference.

Despite its abundance and importance, water is still poorly understood. In fact, more than 100 anomalous properties of water are known, water ice floating on liquid water being the most eye-catching one. © „Iceberg“ Created by Uwe Kils (iceberg) and User:Wiska Bodo (sky). - (Work by Uwe Kils) www.ecoscope.com/iceberg/. Lizenziert unter CC BY-SA 3.0 über Wikimedia Commons - commons.wikimedia.org/wiki/File:Iceberg.jpg
Computing the interactions between electrons and atoms requires solving the laws of quantum mechanics, the Schrödinger equation, accurately enough to capture the fine features. For almost 100 years this has been a very active field of research, in which supercomputing is essential. In fact, the equation is computationally so demanding to solve, that even on the largest supercomputers only approximate solutions can be obtained. However, the resources available at HLRS gave the team lead by Prof. VandeVondele (ETH Zürich) the opportunity to make a significant step forward, enabling the next level of accuracy in such simulations. In particular, computational approaches that treat van der Waals interactions at equal footing with the other forces have been applied to bulk liquid water for the first time. Compared to more standard approaches, these theories require roughly 1000 times more computational power, and have only become possible with significant progress in hard- and software development.[1,2] Interestingly, the weak interactions they describe make all the difference.
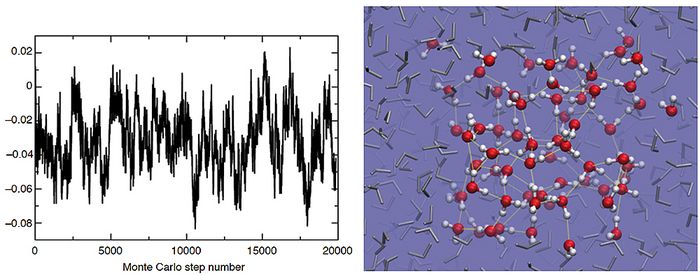
For the first time, Monte Carlo simulations of bulk liquid water (64 H2O) on an RPA potential energy surface can now be performed. Shown is the potential energy evolution, which is characteristic of a well equilibrated system, allowing for a detailed structural analysis. Right: Snapshot of the equilibrated sample of water, with the simulation cell shown embedded in the periodic replicas. Hydrogen bonds, essential for the structure of the liquid and well described by this level of theory, are shown with yellow lines. © M. Del Ben et al. / Computer Physics Communications 187 (2015) 120–129
The results demonstrate convincingly that these advanced simulations methods (named MP2, RPA) yield an accurate structure and density of liquid water.[3,4] Whereas older theories 'predicted' ice to sink in water, finally ice floats! This result represents a breakthrough and enables the use of these theories by computational chemistry teams from all over the world for a broad range of systems. Prof. VandeVondele and his team are currently applying these methods to understand emerging solar cell technology and the electrochemistry that is key for water splitting.
This project was made possible through the Patnership for Advanced Computing in Europe (PRACE). HPC system Hermit of HLRS Stuttgart served as computing platform for this project.
References:
[1] Del Ben M; Schütt O; Wentz T; Messmer P; Hutter J; VandeVondele J; 2015
Enabling Simulation at the Fifth Rung of DFT: Large Scale RPA Calculations with Excellent Time to Solution.
COMPUTER PHYSICS COMMUNICATIONS 187: 120-129
http://dx.doi.org/10.1016/j.cpc.2014.10.021
[2] Del Ben M; Hutter J, VandeVondele J; 2015
Forces and Stress in Second Order Möller-Plesset Perturbation Theory for Condensed Phase Systems within the Resolution-of-Identity Gaussian and Plane Waves Approach
to appear in JOURNAL OF CHEMICAL PHYSICS
http://dx.doi.org/10.1063/1.4919238
[3] Del Ben M; Schönherr M; Hutter J, VandeVondele J; 2013
Liquid water at ambient Conditions from MP2 theory
JOURNAL OF PHYSICAL CHEMISTRY LETTERS 4, 3753-3759
http://dx.doi.org/10.1021/jz401931f
[4] Del Ben M; Hutter J, VandeVondele J; 2015
Submitted.
Please see also: http://www.compchemhighlights.org/2014/01/bulk-liquid-water-at-ambient.html
Scientific contact:
Prof. Dr. Joost VandeVondele
Nanoscale Simulations, ETH Zurich
HIT G 41.4
Wolfgang-Pauli-Strasse 27, CH-8093 Zurich/Switzerland
e-mail: Joost.VandeVondele@mat.ethz.ch
http://www.nanosim.mat.ethz.ch/