MATERIALS SCIENCE AND CHEMISTRY
Single Atom Catalysts Supported on Two-Dimensional Materials for Electrochemical N2 Reduction
Principal Investigator:
Dr. Haobo Li
Affiliation:
Chair for Theoretical Chemistry, TU München, Germany, Current affiliation: School of Chemical Engineering, The University of Adelaide, Australia
Local Project ID:
sacscat
HPC Platform used:
JUWELS at JSC
Date published:
Abstract
Ammonia (NH3) is a versatile compound that finds applications in fertilizer production and fiber manufacturing. The industrial synthesis of ammonia relies on high temperatures and pressures, and requires large amounts of energy while emitting greenhouse gases. A more promising alternative is the electrochemical nitrogen reduction reaction (NRR), which offers a more efficient and environmentally friendly way for ammonia synthesis. Researchers from Technical University of Munich (TUM) have taken this concept further by utilizing artificial-intelligence methods to theoretically design and screen appropriate catalysts for the NRR process. These catalysts, known as single-atom catalysts, play a crucial role in facilitating the reaction.
Introduction
The electrochemical nitrogen reduction reaction (NRR) is a promising process that converts nitrogen gas (N2) into ammonia (NH3) using sustainable electricity under normal conditions. However, the current NRR method has low efficiency and selectivity, leading researchers to search for new catalysts to improve the process.
Nitrogenase naturally converts atmospheric nitrogen into ammonia. It does so on transition metal sulfide (TMS) clusters. The main challenge in improving NRR efficiency lies in the protonation step of the adsorbed *NH intermediate to *NH2, which requires high overpotentials on most TMSs. This step is influenced by scaling relations, which limit the reaction performance on extended TMS catalysts.
To overcome this limitation, researchers focus on constructing specific active site structures, and single-atom catalysts (SACs) are an attractive option due to their isolated active site structure and high atom utilization. However, achieving both high activity and selectivity simultaneously is challenging for SACs on conventional substrates.
Researchers from TUM suggest the combination of TMSs and SACs could be a potential approach to solve this challenge. In this study, a computational screening approach was employed to explore a wide range of such combination systems. A database was created using first-principles density functional theory (DFT) to analyze adsorption energies for NRR intermediates across 27 metals. The artificial-intelligence assisted data analysis revealed strongly broken scaling relations between calculated adsorption energies. Here, supercomputing power plays a key role in helping to build a sufficient database for data mining assisted by artificial-intelligence methods.
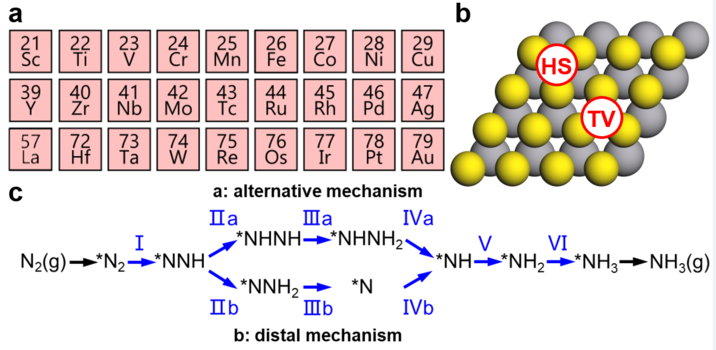
Figure 1. Single-Atom Catalyst Systems and the NRR Reaction Mechanism. © TUM
Results
Further investigation showed that this break of scaling relations was limited to early transition metals and was not solely due to the single-atom nature of the transition metals. Instead, it was found to be a result of charge transfer to the TMS substrate, selectively weakening the metal–N bonds for earlier transition metals. This discovery is significant because earlier transition metals like Ta or W exhibit promising low thermodynamic overpotentials, which positions them favorably on the NRR process.
In summary, researchers are using computational methods and artificial-intelligence to identify catalysts that can improve the efficiency and selectivity of the electrochemical nitrogen reduction reaction. By breaking scaling relations and exploring new catalyst structures, they aim to develop more effective and sustainable methods for future ammonia production.
References and links
Li, H.; Liu, Y.; Chen, K.; Margraf, J. T.; Li, Y.; Reuter, K. ACS Catal. 11, 7906−7914 (2021).
pubs.acs.org/doi/full/10.1021/acscatal.1c01324
Scientific Contact
Dr. Haobo Li
School of Chemical Engineering, The University of Adelaide, Adelaide, SA 5005, Australia.
Email: haobo.li@adelaide.edu.au