MATERIALS SCIENCE AND CHEMISTRY
Force Field Optimization for Ionic Liquids (FFOIL)
Principal Investigator:
Christian Holm
Affiliation:
Institute for Computational Physics, Universität Stuttgart (Germany)
Local Project ID:
FFOIL
HPC Platform used:
Hazel Hen of HLRS
Date published:
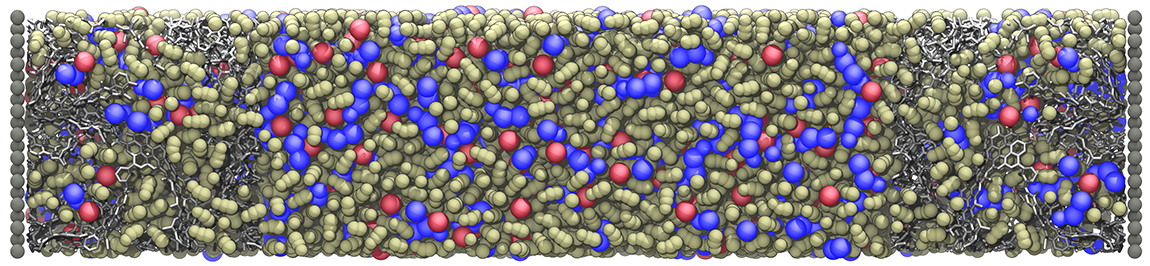
Ionic liquid (IL) based capacitors belong to the class of energy storage devices known as electric double layer capacitors or supercapacitors. They consist of a liquid electrolyte confined between two electrodes of various geometry and material. ILs are known to be good candidates for the dielectric material, as they have advantageous properties for the realization of such a device. In the electrodes, materials with a huge surface area are required in order to achieve high energy densities. Examples for promising electrode materials are graphene sheets, carbon nanotubes or activated carbon, reaching surface areas of more than 1000 m2/g. The outstanding property of supercapacitors is their excellent power density. The (theoretical) absence of chemical reactions and the energy storage mechanisms based on ion separation leads to this low charging time and great cycle stability.
In this context, mixtures of ILs and organic solvents may lead to improved properties in porous electrodes, as the mobility of the charge carriers in highly viscous ILs gets enhanced upon solvation. Detailed Molecular Dynamics Simulations and complimentary experiments study the impact of Acetonitrile solvent concentration in the ionic liquid EMIM+ BF4- confined between nanoporous electrodes.
With intermediate particle numbers of about 15000 atoms, the complexity of this setup lies in the detailed modelling of the metallic electrodes. To analyse capacitance and charging mechanism of the system, the induced charge on each carbon atom on the electrodes is calculated during the entire simulation. These calculations can benefit from high performance computing (HPC), allowing simulations of the dynamic charging process in supercapacitors over tens of nanoseconds with a series of applied voltages and solvent concentrations.
In agreement with experiments, it is shown that (and why) the capacitance is not much affected by dilution of the IL up to a certain concentration, but ion mobility and charging time gets enhanced. These insights may help to optimize the electrolyte mixture for supercapacitor manufacturing.
Scientific Contact:
Konrad Breitsprecher
Institute for Computational Physics, Universität Stuttgart
Allmandring 3, D-70569 Stuttgart (Germany)
e-mail: konrad.breitsprecher [at] icp.uni-stuttgart.de
July 2016