MATERIALS SCIENCE AND CHEMISTRY
In Silico Exploration of Prebiotic Peptide Synthesis by Ab Initio Metadynamics
Principal Investigator:
Dominik Marx
Affiliation:
Lehrstuhl für Theoretische Chemie, Ruhr-Universität Bochum (Germany)
Local Project ID:
hbo27
HPC Platform used:
JUQUEEN of JSC
Date published:
Prebiotic Chemistry is the study of those chemical reactions that could have taken place on the early Earth by which, starting from small molecules like H2O, NH3, CO2, SH2 or simple amino acids, more complex molecules were formed. This leads eventually to the formation of biomacromolecules as we know them from today's life, for instance proteins, RNA or DNA but also lipids. Advanced computer simulations in conjunction with large-scale HPC facilities and scalable codes allow one to investigate at the very molecular level not only how these reactions could have happened, but more importantly how they are affected by factors like temperature, pressure, or the presence of mineral surfaces to name but a few. In this project, the complete set of such reactions leading from amino acids via their activated form to peptides is investigated in a very special environment, namely water at high temperature and pressure conditions in mineral slit pores, which have been suggested to act as chemical nanoreactors for prebiotic synthesis.
Scientists are not sure yet of how or where happened all the chemical reactions leading to the origin of life, and there are many hypotheses for this. One of them, the “Iron-Sulfur World” scenario [1], proposes that iron-sulfur minerals could have catalyzed the necessary reactions that led to the formation of more complex molecules before the emergence of primitive life itself. Of the places where these minerals can be found, deep-sea hydrothermal vents at the bottom of the ocean are probably the most interesting such habitats, since the high temperature and pressure of water therein could have helped the reactions to happen. So far the speculation!
Studying such processes in a 'real' lab is certainly difficult, and even when such experiments can be done, it is often impossible to obtain information at the molecular level. This, however, is a prerequisite to understand reaction mechanisms, which in its turn is required to understand how external factors such as the thermodynamic conditions can either accelerate or decrease the efficiency of the formation process. Scientists set out a decade ago, in 2006, to study such processes on the first Blue Gene/L machine – JUBL – that was available in Germany at Forschungszentrum Jülich. Having access to the respective compute resources, followed by Blue Gene/P (JUGENE) and currently Blue Gene/Q (JUQUEEN) together with ab initio metadynamics sampling [2] and using the powerful CPMD code [3], scientists were able to investigate the influence of extreme temperature and pressure conditions in conjunction with mineral surfaces on peptide bond formation at “Iron-Sulfur World” conditions [4–8]. The simulations disclosed that these specific prebiotic conditions indeed favour the formation of peptide bonds, and thus eventually lead to the stepwise formation of small proteins.
In the current phase of this long-term project, scientists focus on another iron-sulfur mineral called mackinawite. This mineral presents a very interesting feature: it forms layers that are weakly bound and thus it is possible that water intercalates between them forming a very thin film (see figures). As it turns out, water in such situations, called confined water, has different properties from non-confined water, called bulk water, and it is conceivable that the confinement induces changes in these prebiotic reactions.
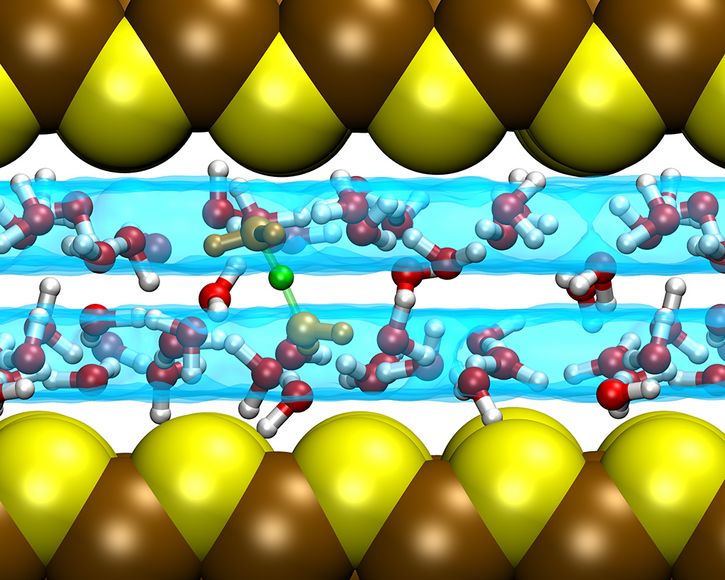
Fig. 01: Confined water in mackinawite is arranged as a double layer, illustrated as the cyan translucent surfaces, in case of a suitable slit pore distance. The excess proton can freely diffuse within each layer and also cross from one to the other, as it is shown here in terms of a green ball being shared between two water molecules (in orange) belonging each to one layer.
Copyright: Lehrstuhl für Theoretische Chemie, Ruhr-Universität BochumNot long ago, this extremely confined pure water was investigated and it was found that the water molecules are able to arrange themselves as a double layer when the spacing between the mineral sheets is appropriate [9]. More recently, it was investigated the simplest chemical reaction, namely proton transfer and diffusion in confined water (see Figure 1). Upon analyzing the mechanism, it was shown that the confinement does not affect much the diffusion properties [10]. In contrast, the hydroxide ion is found to be much more strongly affected by confining alkaline water solutions.
Currently, simulations of the prebiotic reactions leading to the formation of the dipeptide in these water layers in mackinawite slit pores are underway (see Figure 2). Preliminary results suggest that the confinement helps to form peptide bonds and eventually proteins while it protects them from being hydrolyzed at the same time. If confirmed, these results suggest that indeed water lamellae might be a preferential nanoreactor for peptide formation. Moreover, these findings contribute to understand the interplay of the properties of water in extreme confinement and chemical reactivity, which could have very interesting applications that are totally independent from prebiotic chemistry.
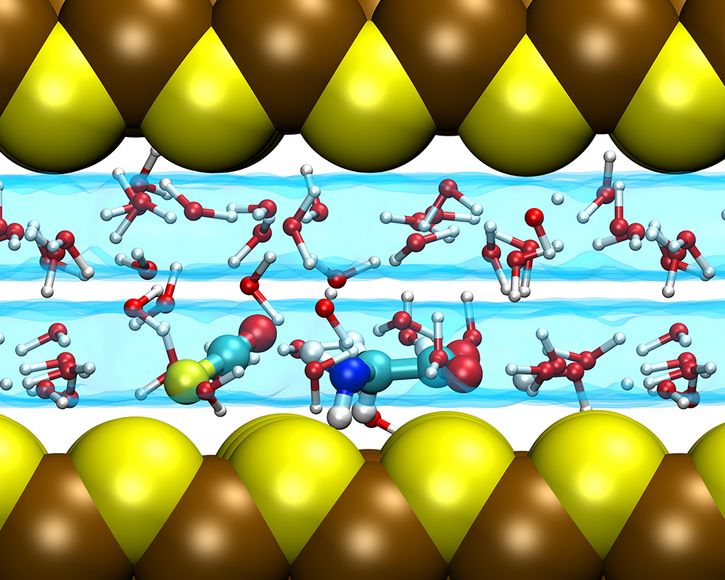
Fig. 02: A molecule of COS, a gas present in hydrothermal vents and required to activate amino acids to form peptide bonds, reacts with an amino acid (glycine) at the beginning of the proposed prebiotic peptide cycle. In the figure it is shown how both molecules are kept close thanks to the confining surfaces, which favors this reaction compared to the same situation in absence of such confinement.
Copyright: Lehrstuhl für Theoretische Chemie, Ruhr-Universität BochumReferences:
[1] Wächtershäuser, G. Groundworks for an evolutionary biochemistry: the iron-sulphur world. Prog. Biophys. Mol. Biol. 58, 85–201 (1992).
[2] Marx, D. & Hutter, J. Ab Initio Molecular Dynamics: Basic Theory and Advanced Methods (Cambridge University Press, Cambridge, 2009).
[3] www.cpmd.org
[4] Nair, N. N., Schreiner, E. & Marx, D. Glycine at the pyrite-water interface: The role of surface defects. J. Am. Chem. Soc. 128, 13815–13826 (2006).
[5] Schreiner, E., Nair, N. N. & Marx, D. Influence of extreme thermodynamic conditions and pyrite surfaces on peptide synthesis in aqueous media. J. Am. Chem. Soc. 130, 2768–2770 (2008).
[6] Nair, N. N., Schreiner, E. & Marx, D. Peptide synthesis in aqueous environments: The role of extreme conditions on amino acid activation. J. Am. Chem. Soc. 130, 14148–14160 (2008).
[7] Schreiner, E., Nair, N. N. & Marx, D. Peptide synthesis in aqueous environments: The role of extreme conditions on peptide bond formation and peptide hydrolysis. J. Am. Chem. Soc. 131, 13668–13675 (2009).
[8] Schreiner, E., Nair, N. N., Wittekindt, C. & Marx, D. Peptide synthesis in aqueous environments: the role of extreme conditions and pyrite mineral surfaces on formation and hydrolysis of peptides. J. Am. Chem. Soc. 133, 8216–8226 (2011).
[9] Wittekindt, C. & Marx, D. Water confined between sheets of mackinawite FeS minerals. J. Chem. Phys. 137, 054710 (2012).
[10] Muñoz-Santiburcio, D., Wittekindt, C. & Marx, D. Nanoconfinement effects on hydrated excess protons in layered materials. Nat. Commun. 4, 2349 (2013).
Scientific team:
Prof. Dr. Dominik Marx (PI) and Dr. Daniel Muñoz-Santiburcio
Scientific contact:
Prof. Dr. Dominik Marx
Lehrstuhl für Theoretische Chemie - Ruhr-Universität Bochum
Universitätsstraße 150, D-44801 Bochum (Germany)
e-mail: dominik.marx@rub.de
June 2016
JSC Project ID: hbo27