MATERIALS SCIENCE AND CHEMISTRY
Kinetic Model of Ethylene Epoxidation on Ag Surfaces
Principal Investigator:
Simone Piccinin
Affiliation:
National Research Council-Istituto Officina dei Materiali (CNR-IOM), Trieste (Italy)
Local Project ID:
PP14102397
HPC Platform used:
Hermit of HLRS
Date published:
Silver’s unique ability to catalyze the partial oxidation of ethylene to ethylene oxide has long been of interest to the scientific community from both a fundamental and practical standpoint. Fundamentally, the reaction represents one of the simplest kinetically controlled partial oxidation, and there is a great drive to understand what factors make silver favor producing ethylene oxide over the thermodynamically favored product, carbon dioxide. From a more practical point of view, ethylene oxide is one of the most important raw materials used by the chemical industry. It is produced entirely over silver. However, even silver is not 100% efficient, and roughly 10% of the ethylene is simply lost in the catalytic process. With tens of millions of tons of ethylene oxide being produced each year, avoiding such a loss is highly desirable.
One of the major challenges in understanding this unique chemistry is identifying how different forms of oxygen on silver react with ethylene. Experimentally such detailed atomic-scale information is hard to come by; thus, researchers turn to computation. To date, computational studies have focused on idealized silver/oxygen structures that are either not known to be present on the catalyst, or are even known not to be present. Part of the reason for this is the complicated structure of known surface species which makes treating them with advanced, accurate, ab initio methods challenging. To overcome this challenge, in the PRACE project “KETHYON - Kinetic model of ethylene epoxidation on Ag surfaces” researchers of the Istituto Officina dei Materiali in Trieste/Italy used the highly parallelizable open source package Quantum ESPRESSO to uncover a realistic picture of the chemistry of ethylene epoxidation. The project was run on the Cray XE6 supercomputer Hermit of the GCS centre High Performance Computing Center Stuttgart.
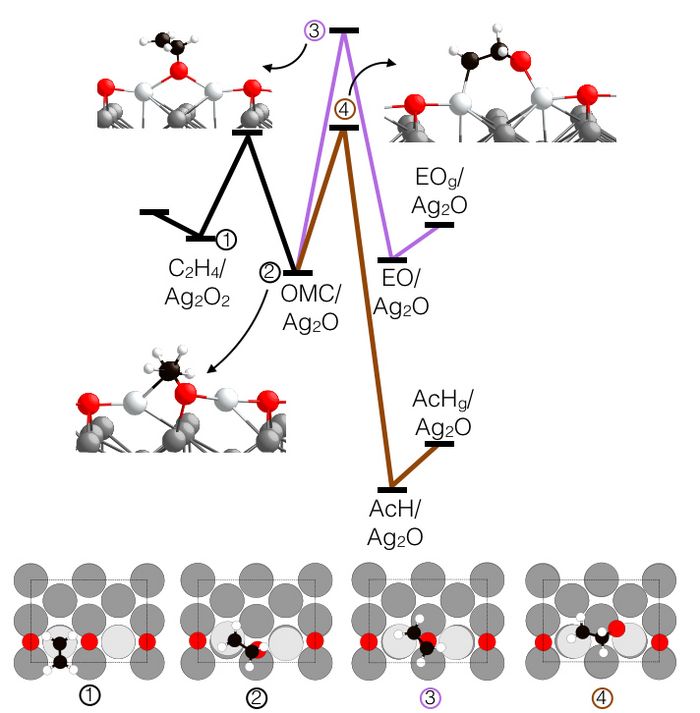
By studying the reaction of ethylene on oxygen induced surface reconstructions on silver, the scientists were able to show that this type of oxygen is not active in epoxide production and strategies should be employed to avoid its appearance under catalytic conditions. For instance, the researchers found that the thermodynamically favored surface phase under ethylene epoxidation conditions on one type of silver surface, the so called p(2x1) reconstruction on the Ag(110) surface, is only active in CO2 production. The reaction diagram below shows that ethylene can bind to the surface (step 1) forming a reactive intermediate (step 2).
At first glance it appears that this intermediate can form the desired product, ethylene oxide, but thorough computational search of plausible reactions reveals that it is much easier for the intermediate to form a different product, acetaldehyde. This other product is then quickly converted into CO2.
Thus, exploiting high performance computing resources unequivocally demonstrated that the thermodynamically favored surface phase under ethylene epoxidation conditions can only burn ethylene. From a practical standpoint, this knowledge makes it worthwhile to consider strategies to avoid the formation of this surface phase. From a more fundamental viewpoint, eliminating the possibility that this stable phase is active in epoxidation allows the community to now shift its attention to identifying unstable oxygen/silver structures that may be responsible for the fascinating chemistry of silver in ethylene epoxidation.
Scientific Contact:
Simone Piccinin
CNR-IOM c/o SISSA
Via Bonomea 265, I-34136 Trieste
e-mail: piccinin [@] iom.cnr.it
April 2016