MATERIALS SCIENCE AND CHEMISTRY
Photocatalytic Water Splitting with Carbon Nitride Materials
Principal Investigator:
Johannes Ehrmaier
Affiliation:
Department of Theoretical Chemistry, Technical University of Munich
Local Project ID:
pr53wo
HPC Platform used:
SuperMUC of LRZ
Date published:
Introduction
Photocatalytic water-splitting, that is, the production of hydrogen and oxygen from water with sunlight, has the potential to provide unlimited, clean and sustainable energy. In 2009, Antonietti and coworkers reported for the first time hydrogen production from water using a carbon nitride (CN) photocatalyst [1]. Since then, CN materials have attracted vast interest in the field. They can split water under irradiation with visible light, consist of earth abundant materials and are photo-stable. In 2015, it was shown that a CN material doped with carbon nano-dots can split water into molecular hydrogen and oxygen in a stoichiometric ratio of 2:1, but the underlying atomistic mechanism is hitherto unknown. Large scale ab initio simulations, including the solvent explicitly, are the only way to gain insight into the photochemical mechanism of water splitting.
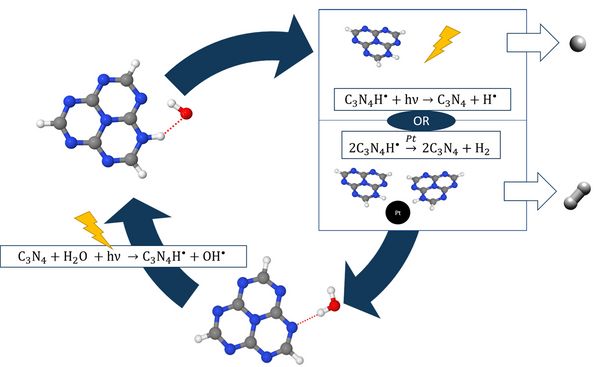
Recently, Domcke and Sobolewski suggested a photochemical mechanism for solar water splitting in hydrogen-bonded molecular systems. [2], [3] The mechanism is summarized in Fig. 1. Absorption of a photon drives an H-atom transfer from water to the catalyst, producing a hydrogenated catalyst radical and an OH radical. Subsequently, the transferred H-atom of the hydrogenated catalyst is either photo-detached and atomic hydrogen is formed, or two hydrogenated chromophore radicals recombine in a dark reaction with the help of a conventional catalyst.
Results and Methods
For this project, carbon nitride systems of different size, from single molecules (triazine and heptazine) to periodic carbon-nitride sheets, were simulated in water to investigate the H-atom transfer reaction from water to the catalyst. The largest systems comprised almost 3000 atoms in total. The catalyst material and the solvent were both treated at the same level of theory. Density functional theory together with the PBE functional was used to equilibrate and to propagate the systems for more than 10 ps. At several snapshots the absorption spectrum as well as properties of the excited states were investigated by time-dependent functional theory. More than 9 Million CPU hours were used to successfully run the calculations.
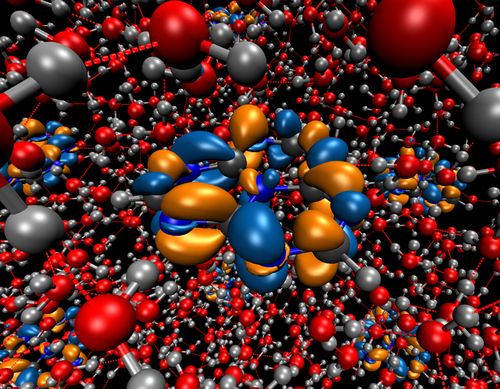
We found that the onset of the spectrum of the catalyst is due to a local ππ* excitation on the catalyst, which only becomes accessible due to the solvation. The transition is dipole forbidden in gas phase. The excitation is at about 2.7 eV, but solvation leads to a width of the energy distribution of about 0.3 - 0.4 eV. Thus, absorption of a photon creates a hole in the valence band of the catalyst and puts an electron in its conduction band. The total density of states of the systems and the projected density of the catalyst were calculated and analyzed. The highest occupied band corresponds to a water orbital. The highest occupied orbital of the catalyst overlaps with the 1b2 band of water. The first unoccupied bands are located on the catalyst and lie in the band gap of water. Thus, a hole on the catalyst can be filled by an electron from water.
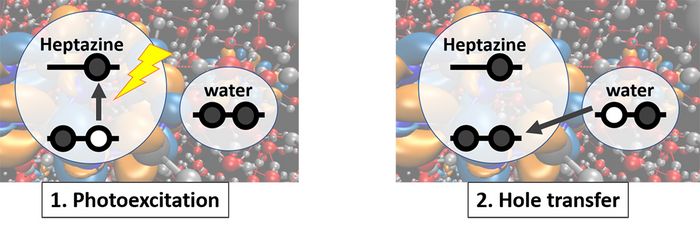
Our simulations give precious insights into the photo-chemistry of the H-atom transfer reaction from water to a carbon nitride material, as proposed by Domcke and Sobolewski. [4] According to the density of states and absorption spectra, we propose an easy picture for an H-atom transfer from water to the catalyst, shown in Fig. 2: The photoexcitation creates a hole in the valence band of the catalyst. This hole can be filled by an electron from water, whose highest occupied bands are energetically above the valence band of the catalyst, creating a hole on water. This electron transfer may drive a proton transfer from water to heptazine, resulting in the hydroxyl heptazinyl biradical.
Research Team
Johannes Ehrmaier (PI), Daniel Opalka (both: Department of Theoretical Chemistry, TU Munich)
References and Links
[1] X. Wang, K. Maeda , A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, “A metal-free polymeric photocatalyst for hydrogen production from water under visible light,” Nature Materials, vol. 8, pp. 76-80, 2009.
[2] J. Liu, Y. Liu, N. Liu, Y. Han, X. Zhang, H. Huang, Y. Lifshitz, S.-T. Lee, J. Zhong and Z. Kang, “Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway,” Science, vol. 347, pp. 970-974, 2015.
[3] J. Ehrmaier, T. N. V. Karsili, A. L. Sobolewski and W. Domcke, "On the Mechanism of Photocatalytic Water Splitting with Graphitic Carbon Nitride: Photochemistry of the Heptazine-Water Complex," J. Chem. Phys. A, vol. 121, no. 25, p. 4754–4764, 2017.
[4] X. Liu, A. L. Sobolewski, R. Borrelli and W. Domcke, “Computational investigation of the photoinduced homolytic dissociation of water in the pyridine-water complex,” Chem. Phys., p. 5957, 2013.
[5] J. Ehrmaier, W. Domcke and D. Opalka, "Mechanism of Photocatalytic Water Oxidation by Graphitic Carbon Nitride," J. Phys. Chem. Lett. 2018, 9, 16, 4695–4699
Scientific Contact
Johannes Ehrmaier
Chair of Theoretical Chemistry
Department of Chemistry
Technical University of Munich
Lichtenbergstraße 4, D-85748 Garching (Germany)
e-mail: johannes.ehrmaier [@] tum.de
NOTE: This report was first published in the book "High Performance Computing in Science and Engineering – Garching/Munich 2018".
LRZ project ID: pr53wo
July 2020