MATERIALS SCIENCE AND CHEMISTRY
Influence of Hydrogen Bonds on Surface Reactions
Principal Investigator:
Thomas Kühne
Affiliation:
Department of Chemistry, University of Paderborn (Germany)
Local Project ID:
hpb01
HPC Platform used:
JUQUEEN (JSC)
Date published:
Researchers at the University of Paderborn currently focus on the further development of the ring polymer path integral molecular dynamics method, and in particular on the simulation of vibrational spectroscopy methods. Leveraging the petascale computing capabilities of HPC system JUQUEEN, they have improved the current understanding of hydrogen bond cooperation by using a proper basis for its description, namely its energy.
Water's ability to form hydrogen bonds is at the core of many of its astounding properties. Cooperation, or cooperativity, describes the notion that a water molecule forms additional hydrogen bonds more readily when it is already hydrogen bonded. However, to this day the intricacies of cooperation in bulk water have not been elucidated as yet.
In this project, run on HPC system JUQUEEN of the Jülich Supercomputing Centre, researchers in the Department of Chemistry at the University of Paderborn under leadership of Prof. Dr. Thomas Kühne have improved the current understanding of hydrogen bond cooperation by using a proper basis for its description: its energy. To this end, they have used data of the energy decomposition analysis of a molecular dynamics simulation of bulk water and the water surface, which has been tuned to represent physical properties of real water at ambient temperature as accurately as possible.
The energy decomposition analysis, ALMO EDA, offers a way to uniquely determine the energies of hydrogen bonds. The scientists detected and quantified a direct dependence of the energy of a hydrogen bond on the energy of other bonds. Apart from that, they elucidated how cooperation and bond energy alters features of two surface specific spectroscopic methods, namely sum-frequency generation and x-ray absorption.
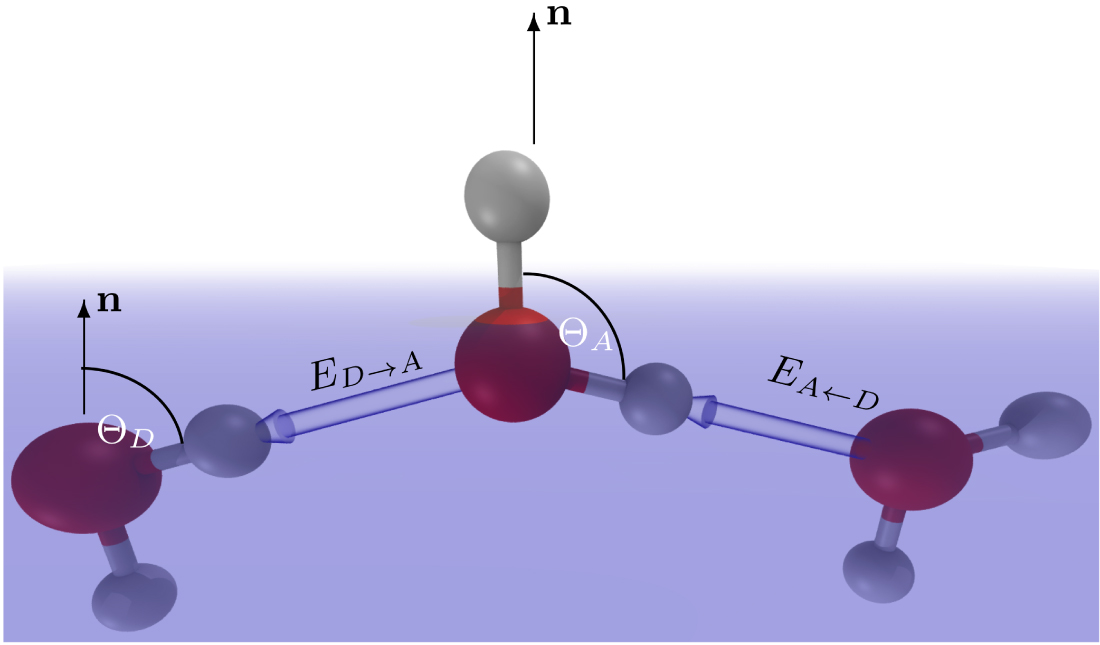
Orientation of the water molecules at the water/vapor surface.
Copyright: University of Paderborn, Department of ChemistryScientific Team:
Kristof Karhan, Hossam Elgabarty, Pouya Partovi-Azar, Deepak Ojha, Andres Henao
References:
T. D. Kühne and R. Z. Khaliullin, J. Am. Chem. Soc. 136, 3395 (2014).
K. Karhan, R. Z. Khaliullin and T. D. Kühne, J. Chem. Phys. 141, 22D528 (2014).
H. Elgabarty, R. Z. Khaliullin and T. D. Kühne, Nature Commun. 6, 8318 (2015)
J. Kessler, H. Elgabarty, T. Spura, K. Karhan, P. Partovi-Azar, A. A. Hassanali and T. D. Kühne, J. Phys. Chem. B 119, 10079 (2015).
T. Spura, C. John, S. Habershon and T. D. Kühne, Mol. Phys. 113, 808 (2015).
P. Partovi-Azar and T. D. Kühne, J. Comp. Chem. 36, 2188 (2015).
C. John, T. Spura, S. Habershon and T. D. Kühne, Phys. Rev. E 93, 043305 (2016).
D. Ojha, A. Henao and T. D. Kühne, J. Chem. Phys. 148, 102328 (2018)
Scientific Contact:
Prof. Dr. Thomas Kühne
Department of Chemistry - Technical Chemistry
University of Paderborn
Warburger Straße 100, D-33098 Paderborn (Germany)
tdkuehne [at] mail.uni-paderborn.de